Back to top

Certifications
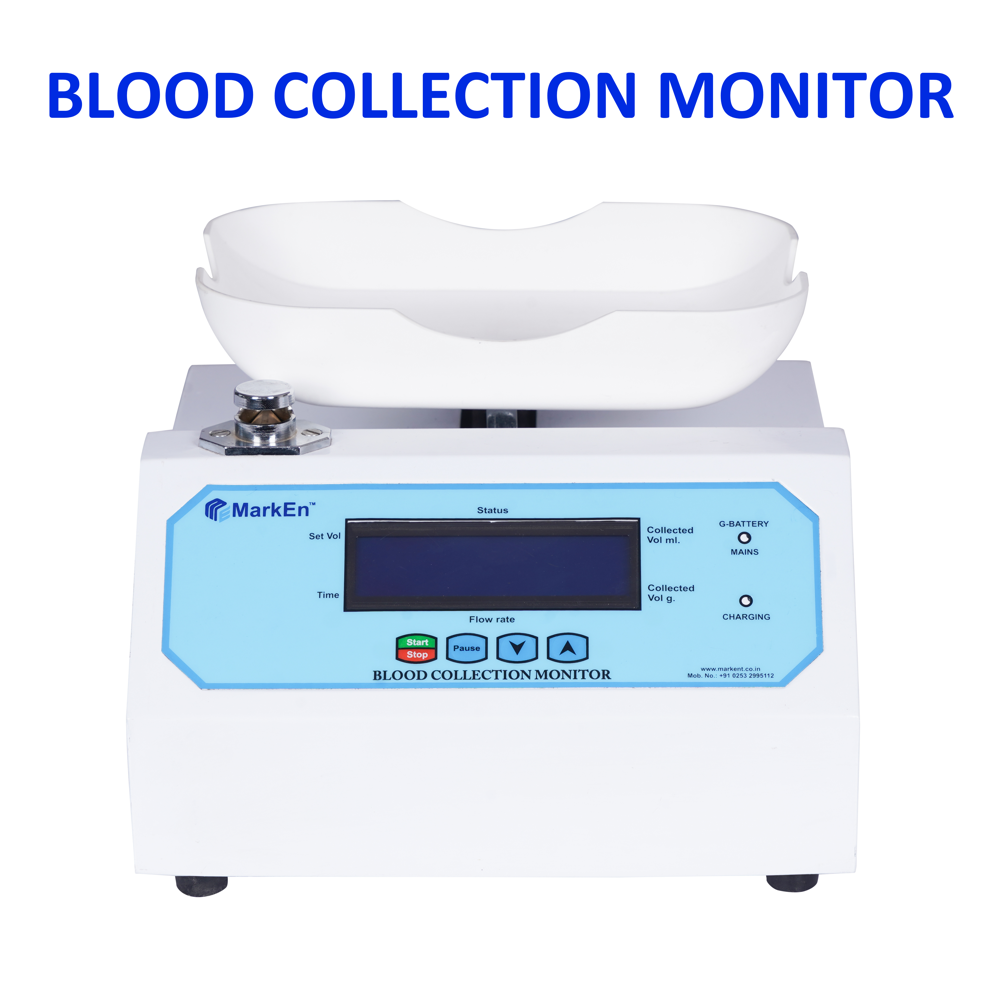
Best in the market to buy reliable Solar Direct Drive Refrigerator, Laminar Air Flow, Gel Card Incubator, Ice Lined Refrigerator, Ultrasonic Cleaner, Pediatric Bed, Blood Bank Refrigerator, Laboratory Autoclave, etc.
Marken Healthtech Private Limited was formed in 2018 with the name Mark Enterprises. Since inception, we have been passionately running our operations with a vision to provide easily accessible and dependable Cold Chain Solutions for the Healthcare Sector. We have become a renowned manufacturer and exporter of Solar Direct Drive Refrigerator, Ice Lined Refrigerator, Blood Bank Refrigerator, Laminar Air Flow, Gel Card Incubator, Laboratory Autoclave, Ultrasonic Cleaner, Pediatric Bed, etc.
We perform our job duties with an unwavering commitment to quality and focus on gaining complete satisfaction of customers. This has earned us recognition and accreditation from many renowned names of the market, including the All State Governments, Central government of India, Research Institutes, Defense forces, and more. We design our entire range of products by keeping in mind the need for a comprehensive ecosystem in Medical Cold Chain Equipment and the Healthcare Sector. Furthermore, we render dependable after sales services for all the offered products.
We perform our job duties with an unwavering commitment to quality and focus on gaining complete satisfaction of customers. This has earned us recognition and accreditation from many renowned names of the market, including the All State Governments, Central government of India, Research Institutes, Defense forces, and more. We design our entire range of products by keeping in mind the need for a comprehensive ecosystem in Medical Cold Chain Equipment and the Healthcare Sector. Furthermore, we render dependable after sales services for all the offered products.
Unique Selling Points
- 1st in World to launch Programmable Remote Temperature and Event Monitoring System
- 6th in World to have Walk-In-Cooler
- 3rd in India to have Deep Freezer (small)
- 200 Cr Funding By Government to contribute to Make in India campaign
- 25 Products and 5 years of experience in the Medical Equipment industry
- 48 crores subsidy by Government
Certifications
|
|

















 Send Inquiry
Send Inquiry

